Classification of Drugs: Understanding Drug Schedule I-V or Schedule 1-5
Summary: Drug schedules are a classification system used by the U.S. Drug Enforcement Administration (DEA) to categorize controlled substances into five categories (Schedule I through Schedule V) based on their accepted medical use, potential for abuse, and risk of physical or psychological dependence. Schedule I substances like heroin and LSD have no accepted medical use and the highest abuse potential, while Schedule V substances like low-dose codeine preparations carry the lowest risk. At Overland IOP in Los Angeles, our medication management program works within these classifications to ensure safe prescribing, monitoring, and tapering as part of comprehensive addiction and mental health treatment.
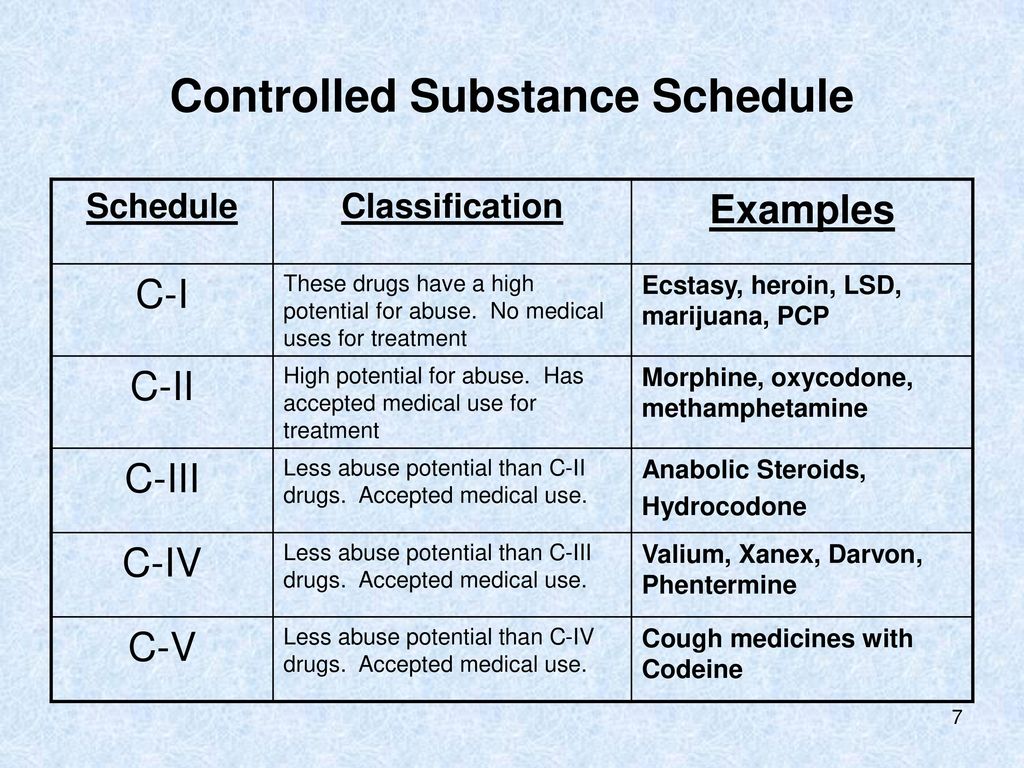
U.S. controlled substances are grouped into five “schedules” that balance medical use with risks for abuse and dependence. Schedule I substances have no accepted medical use federally, while Schedules II–V do, with progressively lower abuse potential and lighter prescribing rules. Knowing where a medication sits helps explain why refills, ID checks, and pharmacy rules can feel strict—and how Overland IOP keeps patients safe.
What “drug schedule” means
The Drug Enforcement Administration (DEA) places drugs into Schedules I–V based on medical use, abuse potential, and risk of dependence. This classification drives how prescribers write, pharmacies dispense, and records are kept for safety and diversion prevention.
When it comes to understanding how drugs are regulated, knowing how they’re classified is key. In the world of pharmacology, drugs aren’t all treated equally —they’re divided into schedules based on factors like their medical use, potential for abuse, and risk of dependency.
These drug schedules (ranging from Schedule I to Schedule V) help shape how substances are prescribed, dispensed, and monitored. From the most tightly controlled to the least, each category plays an important role in balancing access to medications with public safety.
Schedule I Drugs or Schedule 1 Drugs
Schedule 1 drugs are classified as substances with a high potential for abuse and no recognized medical use. These drugs are strictly regulated and illegal in most jurisdictions. Examples of Schedule 1 drugs include heroin, LSD, and ecstasy (MDMA). Due to their addictive properties and severe health risks, these substances are strictly prohibited and subject to stringent legal penalties.
Note: It is important to acknowledge that, while marijuana is classified as a Schedule 1 drug at the federal level in the United States, its legal status varies at the state level. Several states have legalized marijuana for medical and/or recreational use, which may create variations in its legal status and accessibility across different jurisdictions. It is essential to consult local laws and regulations regarding marijuana use and distribution in specific regions.
Schedule II Drugs or Scheduele 2 Drugs
Schedule 2 drugs possess a high potential for abuse but have accepted medical uses. They are subject to strict regulations, including limitations on prescriptions and dispensing. Substances in this schedule include powerful opioids such as oxycodone, morphine, and fentanyl, as well as stimulants like amphetamines. Due to their potential for addiction and misuse, these drugs are highly controlled and require careful monitoring and oversight.
Schedule III Drugs or Schedule 3 Drugs
Schedule 3 drugs have a moderate potential for abuse but still possess accepted medical uses. Schedule 3 drugs require a prescription for legal acquisition, but the regulations governing them are relatively less stringent compared to those for Schedule 2 drugs. Examples of Schedule 3 substances include certain opioids with lower abuse potential, such as codeine preparations, as well as some anabolic steroids. These medications are subject to prescribing restrictions and monitoring to prevent abuse and ensure patient safety.
Schedule IV Drugs or Scheduele 4 Drugs
Schedule 4 drugs, also known as prescription-only medicines, have a moderate potential for abuse but hold significant therapeutic value. These drugs require a prescription from a healthcare professional for lawful acquisition and use. Schedule 4 substances include medications such as benzodiazepines (e.g., diazepam), certain opioids like tramadol, and sleep aids like zolpidem. While they have recognized medical uses, their potential for abuse necessitates regulation and controlled distribution.
Schedule V Drugs or Scheduele 5 Drugs
Schedule 5 drugs are classified as medications with a low potential for abuse or dependence. They have accepted medical uses and can be purchased without a prescription, albeit with some regulations in place. Schedule 5 substances have the lowest potential for abuse among controlled substances and are commonly used to treat mild conditions. Examples include medications containing low-dose codeine, diphenoxylate/atropine combinations, and certain antidiarrheal agents. While relatively safe, these drugs still require responsible use and adherence to recommended dosages.
Other Schedules & State-by-state differences
In addition to the schedules mentioned above, some countries or regions may have further classifications for controlled substances. These schedules are often jurisdiction-specific and aim to regulate substances based on their properties, potential for abuse, and medical uses. These additional schedules, such as Schedule 6 and higher, provide further categorization and legal frameworks specific to particular regions.
States can add controls or create additional categories beyond federal schedules, so availability and procedures (ID, quantity, pharmacist steps) may differ locally. Always follow your prescriber’s and pharmacist’s guidance.
Conclusion
Proper classification of drugs into different schedules provides a fundamental framework for regulating controlled substances based on their properties, potential for abuse, and medical uses. Understanding the distinctions between Schedule 1, Schedule 2, Schedule 3, Schedule 4, and Schedule 5 drugs is crucial for healthcare professionals, law enforcement agencies, policymakers, and individuals involved in the field of pharmacology.
FAQs: Classification of Drugs: Understanding Drug Schedule I-V or Schedule 1-5
FAQ1: What’s the single biggest difference across schedules?
The five drug schedules are categories established by the DEA to classify controlled substances. Schedule I includes drugs with no accepted medical use and high abuse potential (e.g., heroin, LSD). Schedule II includes drugs with accepted medical use but high abuse risk (e.g., oxycodone, fentanyl, Adderall). Schedule III covers moderate-risk drugs like codeine combinations and anabolic steroids. Schedule IV includes lower-risk prescriptions like benzodiazepines and tramadol. Schedule V contains the lowest-risk controlled substances, such as cough syrups with small amounts of codeine.
FAQ2: Why can’t I refill my Schedule II prescription?
Federal law prohibits refills of Schedule II drugs; a new prescription is required each time. Your prescriber may issue multiple separate prescriptions to cover up to a 90-day supply when appropriate. DEA Diversion Control Division
FAQ3: What is the difference between Schedule I and Schedule II drugs?
The key difference is accepted medical use. Schedule I drugs like heroin and ecstasy have no recognized medical use under federal law and cannot be prescribed. Schedule II drugs like oxycodone, morphine, fentanyl, and amphetamines have accepted medical uses but carry a high potential for abuse and dependence, requiring strict prescribing controls and no refills without a new prescription.
FAQ4: What about Schedule V refills?
Schedule V refills are allowed as authorized by the prescriber; state rules can add limits, so your pharmacy may advise specifics. PMC
FAQ5: Why is marijuana classified as a Schedule I drug?
Marijuana remains classified as a Schedule I substance under federal law, meaning it is considered to have no accepted medical use and a high potential for abuse at the federal level. However, many states have legalized marijuana for medical and recreational use. A federal proposal to reschedule cannabis to Schedule III has been under review but has not been finalized. State and federal laws may conflict, so it is important to follow local regulations..
FAQ6: Why is tramadol listed with Schedule IV?
Tramadol is an opioid pain medication that was originally marketed in the U.S. without controlled substance status. However, after evidence of widespread misuse emerged — with nearly 40 million prescriptions written in 2012 alone — the DEA issued a final rule on July 2, 2014, placing tramadol into Schedule IV of the Controlled Substances Act, effective August 18, 2014. The Department of Health and Human Services recommended this classification after evaluating tramadol’s abuse potential, dependence liability, and opioid-like pharmacological effects through its active M1 metabolite. As a Schedule IV substance, tramadol now requires a prescription, DEA registration for handlers, and compliance with all Schedule IV dispensing, labeling, and record-keeping rules. DEA Final Rule — Placement of Tramadol Into Schedule IV
FAQ7: Is pregabalin really controlled?
Yes. Pregabalin (brand name Lyrica) was placed into Schedule V of the Controlled Substances Act effective July 28, 2005, shortly after the FDA approved it for marketing in December 2004. The DEA’s decision was based on an evaluation by the Department of Health and Human Services showing that pregabalin can produce euphoria and positive subjective effects — particularly in patients with a history of substance abuse — though its overall abuse potential is lower than Schedule IV benzodiazepines. Pregabalin is widely prescribed for neuropathic pain, fibromyalgia, and epilepsy, but as a Schedule V controlled substance it still requires a prescription and compliance with controlled substance dispensing rules. DEA Final Rule — Placement of Pregabalin Into Schedule V
FAQ8: Do drug schedules change?
Yes — the DEA can reschedule, add, or remove substances based on new scientific and medical evidence. The process involves an evaluation by the Department of Health and Human Services, a notice of proposed rulemaking published in the Federal Register, a public comment period, and a final rule. Recent examples include tramadol being added to Schedule IV in 2014, FDA-approved cannabidiol (Epidiolex) being placed in Schedule V in 2018, and a 2023 rule allowing electronic prescription transfers between pharmacies for Schedules II–V. When a substance’s schedule changes, all prescribing, dispensing, refill, and record-keeping rules update accordingly. DEA Drug Scheduling Overview | DEA Rule — E-Prescription Transfers (2023)
FAQ9: How do drug schedules affect my treatment at Overland IOP?
A medication’s schedule classification directly shapes how our clinical team designs and manages your treatment plan. Higher-schedule medications (Schedule II–III) require more structured tapering protocols, closer monitoring for dependence, and more frequent coordination with your prescriber and pharmacy — including new prescriptions for each fill since refills are prohibited for Schedule II drugs. For lower-schedule medications (IV–V), our team monitors for misuse while maintaining appropriate therapeutic access. At Overland IOP in Los Angeles, our medication management program integrates pharmacological interventions with evidence-based therapies like CBT and DBT to support safe, effective recovery while minimizing risk of substance misuse.

Prescription medication management is one of our valuable services at Overland IOP, a mental health and addiction treatment center. This service recognizes the significance of adhering to the appropriate drug schedules while ensuring the safe and responsible use of medications. By offering comprehensive treatment plans that encompass pharmacological interventions, Overland IOP aims to optimize patient care and promote long-term recovery. The classification of drugs by schedule serves as an important tool in promoting patient safety, preventing substance abuse, and facilitating appropriate access to medications. Through responsible prescribing practices, monitoring, and education, we can harness the power of pharmacology to improve lives and support individuals on their journey toward health and well-being.
Published: July 14, 2023
Last Updated: February 23, 2026

Published: February 14, 2026
Medication-Assisted Treatment (MAT): How It Works?
Summary: Medication-assisted treatment (MAT) is an evidence-based approach to addiction treatment that combines FDA-approved medications with behavioral therapy and counseling to treat substance use disorders — primarily opioid and alcohol addiction. MAT is endorsed by the Substance Abuse and Mental Health Services Administration (SAMHSA), the National Institute on Drug Abuse (NIDA), and the World Health […]
Read more
Published: February 06, 2026
Talk Therapy: Types, Benefits & How It Works in California
Summary: Talk therapy — also known as psychotherapy — is a structured, evidence-based treatment approach in which a trained mental health professional helps individuals identify, understand, and change the thoughts, emotions, and behaviors that contribute to mental health conditions and substance use disorders. It is the foundation of treatment for depression, anxiety, PTSD, personality disorders, […]
Read more
Published: January 27, 2026
What Is DPD? Understanding Dependent Personality Disorder
Most people don’t ask, “What is DPD or Dependent Personality Disorder?” They come in feeling drained, anxious, and stuck in relationships that feel restrictive yet hard to leave. Being alone feels unsettling. Decision-making feels paralyzing. Reassurance becomes a daily necessity rather than a comfort. At Overland IOP in Los Angeles, we often see Dependent Personality […]
Read more
